BIOMECHANICS
The Alemnis Standard Assembly (ASA) is perfectly adapted to the testing of biomaterials in a range of environments, both in-situ and ex-situ. Some examples include bone micropillars (made from FIB milling) tested inside the SEM, submerged biomaterials tested ex-situ in a liquid cell and analysis of biocompatible coatings for dental applications.
The Liquid Cell (LIC) can be used for a wide range of experiments where the sample needs to be submerged in liquid. It is therefore typically used ex-situ with the Alemnis Standard Assembly (ASA) mounted vertically with the LIC mounted on the load cell. Some examples of how the LIC can be used are:
- Submerging biomaterials in saline solution or Body-Mimicking Fluid (BMF) to evaluate their mechanical properties when hydrated
- Electro-chemical tests where the indenter may be used as a current probe and the electrolyte (liquid) may be activated using an external potentiostat for corrosion or passivation studies
- Studies of hydrogel meso-porous effects, i.e. how the mechanical properties change as a function of the level of hydration.
- Tribological studies of interaction between the indenter and the sample when submerged in liquid. Often used for simulating prosthetic joints.

Vertically mounted ASA with liquid cell (LIC) for making tests in liquid
The Relative Humidity Module (RHM) is an environmental sample chamber to allow mechanical tests to be performed under conditions of controlled humidity, temperature, or in liquid (sample fully submerged). The typical configuration of the ASA is vertically mounted with the indenter above the sample, but other configurations are also possible depending on options installed.
The relative humidity (RH) can be controlled over the range 5 – 95% with an accuracy of 1.5%. The temperature range is from room temperature to 70°C with an accuracy 0.1°C.
The environmental sample chamber consists of:
– the environmental chamber with all mounts;
– all cables, connectors, pipes;
– controller for relative humidity and temperature closed loop control.
For fully submerged testing without RH and temperature control, the Liquid Cell (LIC) can be used.

The Bio-indenter is an ex-situ variant of the Alemnis Standard Assembly (ASA) which is specifically designed for characterizing the mechanical properties of tissues and soft materials. It can be configured with the Relative Humidity Module (RHM) and the Miniaturized Load Cell (MLC) to provide a complete testing platform for a wide variety of sample configurations (micropillars, particles, gels, tensile testing, etc..) in a range of environments (controlled relative humidity and temperature, submerged in liquid, etc..). The basic specifications are as follows:
Applied load range: 10 µN – 500 mN (by choosing a different load sensor, the maximum range can be extended up to 4 N)
Load noise floor: 4 µN
Displacement range: up to 100 µm (using DHP-100)
Displacement noise floor: 1 nm
The Bio-indenter configuration can also be coupled with an optical microscope via a piezo-actuated XY displacement stage. The optical microscope has the following specifications:
- Objective with zoom (0.77 – 9.31x)
- Working distance 86mm
- Color camera 1280 x 1024 pixel
- Coaxial and annular illumination
The Bio-indenter has proved itself for many ground-breaking scientific studies:
Micropillar compression of hydrated lamellar bone at high strain rates
Micropillar compression experiments on sheep bone over a wide range of strain rates (10−4 to 8·102 s−1), at two different orientations, and under humid condition to mimic the natural conditions of bone in a body revealed a microscale strain rate sensitivity similar to what is reported at the macroscale. This study highlights the importance of investigating bone strength and post-yield behavior at lower length scales, under hydrated conditions and at clinically relevant strain rates. (Ref. 9)
Tensile Testing at the Microscale
Tensile properties of bone extracellular matrix were evaluated in both axial and transverse orientations on the length scale of a single lamellae. Tensile test specimens were fabricated by focused ion beam (FIB) milling of ovine osteonal bone and two distinct failure modes were identified from in-situ observation and post-failure high resolution scanning electron micrographs.
Casari et al, World Congress of Biomechanics, Dublin, 2018
Micropillar compression of wood
A good example of how the Bio-indenter can be used is the example of Ref. 4 which describes micropillar compression of wood (Norway Spruce) samples made by FIB milling. The hierarchical architecture of wood makes it an interesting candidate for structure-property investigation over many length scales. The stem of softwoods features a characteristic pattern of early and latewood forming the so-called growth rings which consist mainly of tracheid cells which are rectangular or circular hollow tubes aligned with the length axis of the stem. In the tracheid cell wall (cw), semi-crystalline cellulose (amorphous cellulose (ac) and crystalline cellulose (cc)) microfibrils are embedded in a polymeric network of hemicellulose (hc) and lignin (li) and aligned parallel to each other similar to the situation in a unidirectionally fibre-reinforced composite. The fibrils are inclined at an angle to the cell axis called the microfibril angle (MFA) leading to a helical structure.
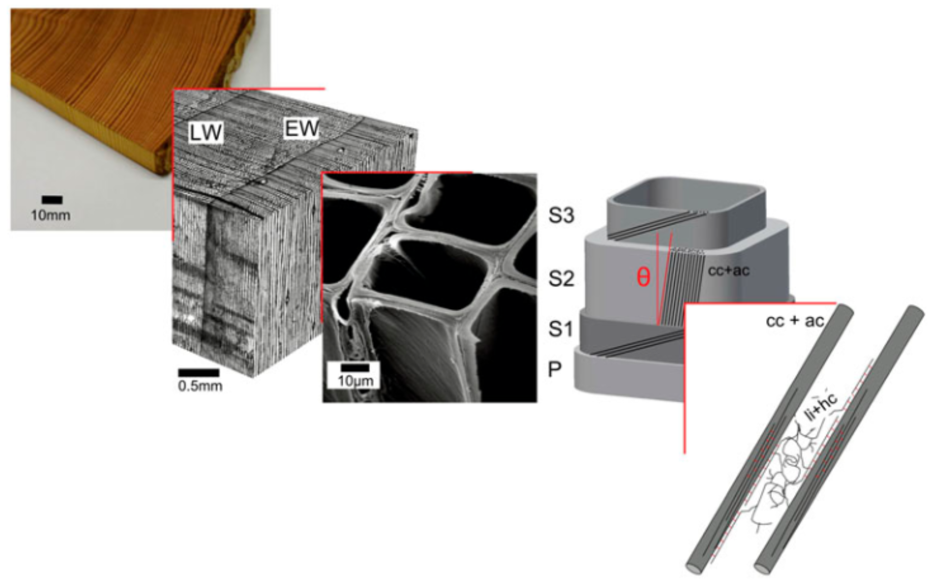
Hierarchical structure of wood from the macro to the nanoscale consisting of amorphous cellulose and crystalline cellulose, hemicellulose and lignin (from Ref. 4)
Although nanoindentation has been used to study wood properties, the interpretation of the data remains problematic owing to the non-homogeneous structure and the local inelastic deformation under the indenter. Therefore, micropillar compression experiments with well-defined boundary conditions and a homogeneous stress state provide a more straightforward interpretation of elastic behavior in wood at the cell wall level and below. In combination with X-ray diffraction and wet chemical analysis, the micropillar compression technique has allowed differences in mechanical behavior between two types of wood tissue (normal and compression) to be explained and the shear yield stress of the polymer matrix and lignin to be measured.
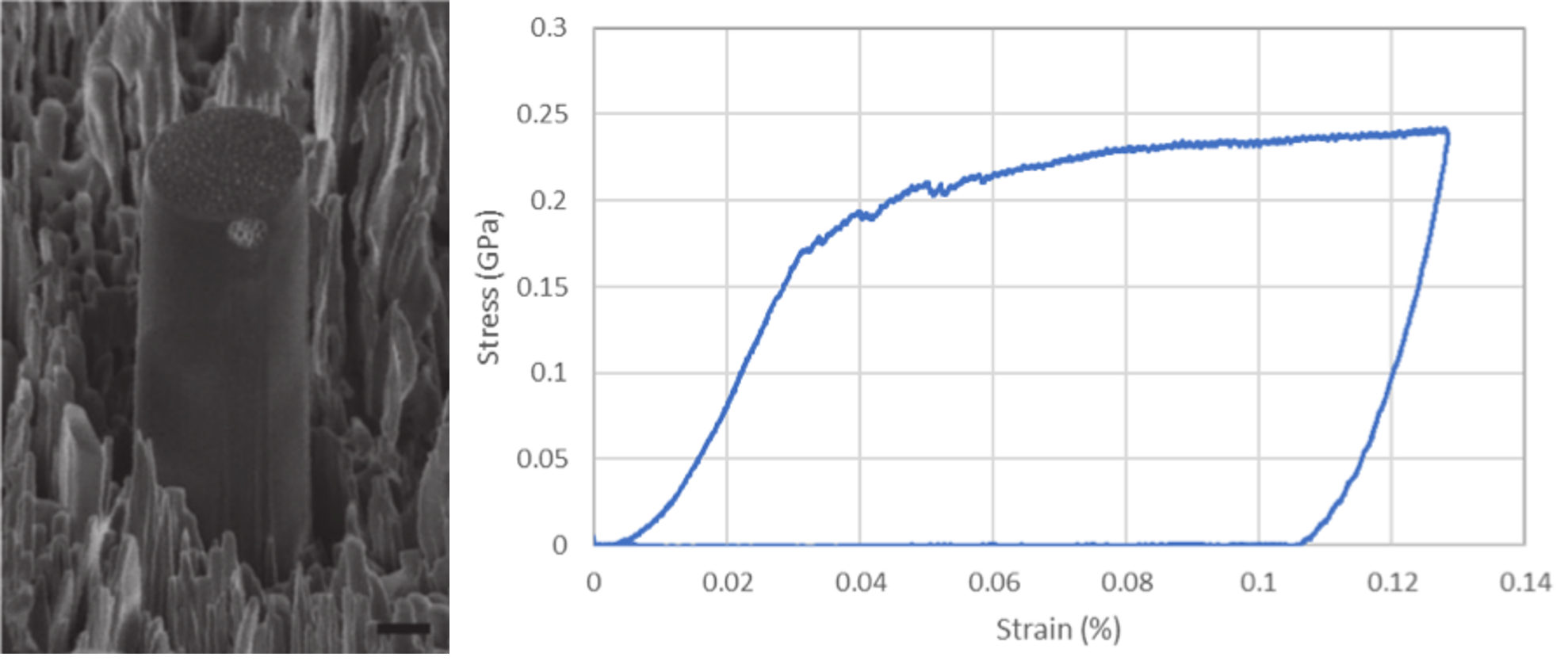
Typical example of micropillar produced by FIB-milling of Norway spruce wood with corresponding stress-strain curve. Scale bar represents 1 µm (From Ref. 4)
Micropillar compression of collagen fibres combined with X-ray scattering (SAXS) and X-ray diffraction (XRD)
This application of the ASA has allowed micropillar compression data to be related to the mechanical behavior of individual mineralized collagen fibres in an effort to better predict fracture risk in osteoporotic bone fractures. The extraction of micropillars from individual mineralized collagen fibres is achieved by first dissecting flexor tendons from the lower parts of turkey legs. The resulting tendon pieces were then dried at room temperature for 24h, glued to a SEM stub and ultra-short pulsed laser ablation used to cut pre-pillars of 33 µm diameter and 50 µm height, centred around a single mineralized collagen fibre. Focused ion beam (FIB) milling was then used to machine the micropillars down to a final diameter of 6.0-7.5 µm and height of approximately 13 µm. Samples were then sputtered with gold prior to compression testing.
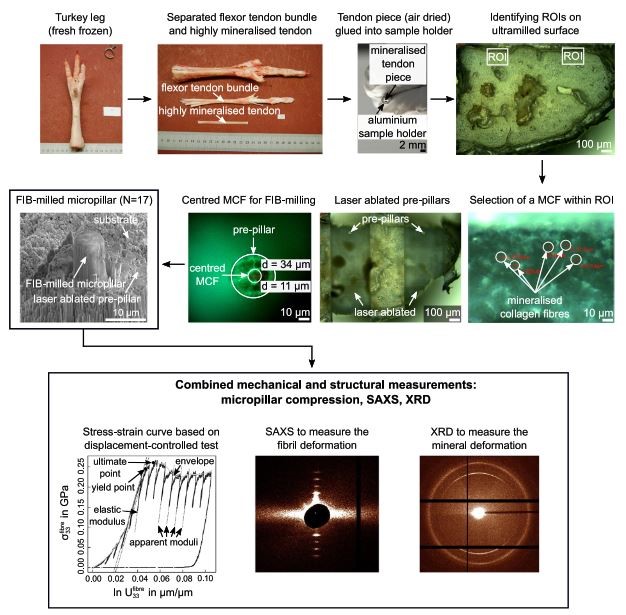
Summary of preparation steps required to extract individual mineralized collagen fibres by means of dissection, ultra-milling, ultra-short pulsed laser ablation and focused ion beam milling. The SEM image shows the final micropillar which was compression tested whilst acquiring small angle X-ray scattering (SAXS) and X-ray diffraction (XRD) data (from Ref. 7)
The Alemnis Standard Assembly (ASA) was installed at the microfocus beamline ID13 of the European Synchrotron Radiation Facility (ESRF) in Grenoble, France to allow simultaneous micropillar compression tests and SAXS or XRD acquisitions. After sample mounting, the ASA was controlled remotely from the beamline hutch to align it with the beam path and the detector using the beamline’s sample stage. The flat punch indenter was aligned with a micropillar using the optical microscope and the X axis of the ASA.
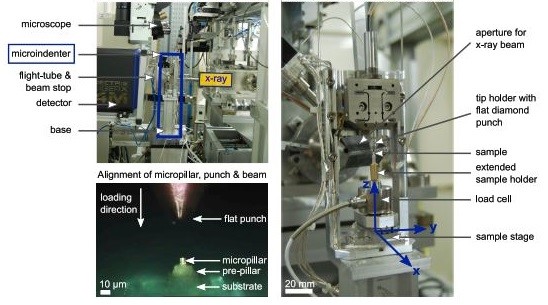
Detail of how the ASA is integrated into the ID13 beamline at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France. The top left image shows the detector in XRD position (from Ref. 7)
The micropillars were compressed uniaxially in a displacement-controlled test to 1.5 µm deformation at 5 nm/s including 50 nm partial unloading steps every 150 nm, while recording load, displacement and time data. Two groups of samples were then used to measure either SAXS or XRD to obtain the deformation of the mineralized collagen network (fibril strain; strain in the calcified collagen phase) and mineral nanocrystals (mineral strain; strain in the mineral phase) respectively.
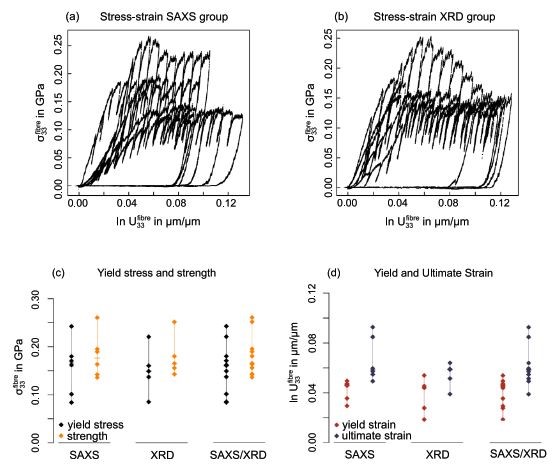
Summary of mechanical properties of the tested fibre micropillars, after correction of sink-in and frame compliance, for 2 groups (SAXS and XRD) (from Ref. 7)
Stress and strain curves at the fibre level showed the same characteristic development as reported elsewhere for micropillar compression tests on osteonal bone. Compared to dry axial micropillar compression tests on ovine bone and bovine bone, the compressive strength was 3 to 4 times smaller but the ultimate strain 2.5 times larger. Differences in these absolute values can be associated with the different structural setup of the fibrils in bone tissue which is tailored towards compression whereas tendon tissue is tailored towards tension. In addition, different degrees of mineralization of both tissues need to be considered.
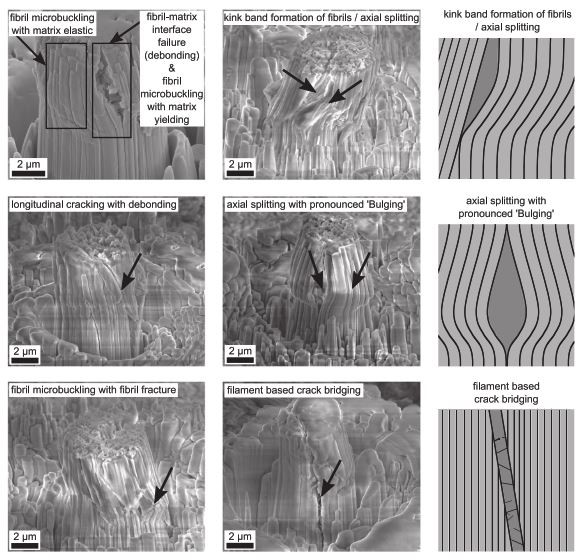
Failure modes and patterns of compressed micropillars with thick black lines representing fibrils, thin black lines filaments and darker areas failure zones (from Ref. 7)
The presented data demonstrates the importance and the benefits when testing mineralized tissue at the length scale of their fundamental mechanical building blocks which gives novel insights into the tissue’s non-linear mechanical behavior at the level of the mineralized collagen fibre. The deformation behavior within an individual mineralized collagen fibre thus provides valuable input for micromechanical models and computational non-linear bone strength analyses optimizing approaches for fracture risk prediction. Eventually, such results aim to improve personalized diagnosis and treatment solutions as well as bio-inspired implants for people with bone diseases.
Selected References
- Schwiedrzik J, Raghavan R, Bürki A, LeNader V, Wolfram U, Michler J, et al. In situ micropillar compression reveals superior strength and ductility but an absence of damage in lamellar bone, Nature Materials 13 (2014) 740-747
- Lunt AJ, Mohanty G, Ying S, Dluhoš J, Sui T, Neo TK, et al. A comparative transmission electron microscopy, energy dispersive x-ray spectroscopy and spatially resolved micropillar compression study of the yttria partially stabilised zirconia-porcelain interface in dental prosthesis, Thin Solid Films 596 (2015) 222-232
- Lunt AJ, Mohanty G, Neo TK, Michler J, Korsunsky AM. Microscale resolution fracture toughness profiling at the zirconia-porcelain interface in dental prostheses, SPIE Micro+ Nano Materials, Devices, and Applications: International Society for Optics and Photonics; 2015. p. 96685S-S-11.
- Schwiedrzik J, Raghavan R, Rüggeberg M, Hansen S, Wehrs J, Adusumalli RB, et al. Identification of polymer matrix yield stress in the wood cell wall based on micropillar compression and micromechanical modelling, Philosophical Magazine 96 (2016) 3461-3478
- A. Groetsch, A. Gourrier, J. Schwiedrzik, M. Sztucki, J. Shephard, J. Michler, P. K. Zysset, U. Wolfram, Simultaneous micropillar compression and X-ray scattering or diffraction to investigate scale effects of strains in mineralised collagen fibres, Paper presented at 12th International Conference on Advances in Experimental Mechanics, Sheffield, United Kingdom (2017)
- Ferrand, H.L., F. Bouville, and A.R. Studart, Processing of dense bio-inspired ceramics with deliberate microtexture, arXiv preprint arXiv:1807.04378 (2018)
- A. Groetsch, A. Gourrier, J. Schwiedrzik, M. Sztucki, R. J. Beck, J. D. Shephard, J. Michler, P. Zysset and U. Wolfram, Compressive behavior of uniaxially aligned individual mineralized collagen fibres at the micro- and nanoscale, Acta Biomaterialia 89 (2019) 313-329
- H. D. Espinosa, A. Zaheri,H. Nguyen,D. Restrepo, M. Daly, M. Frank & J. McKittrick, In situ wear study reveals role of microstructure on self-sharpening mechanism in sea urchin teeth, Matter 1(2019) 1-16
- Peruzzi, C., Ramachandramoorthy, R., Groetsch, A., Casari, D., Grönquist, P., Rüggeberg, M., Michler, J., & Schwiedrzik, J. (2021). Microscale compressive behavior of hydrated lamellar bone at high strain rates. Acta Biomaterialia, 131, 403–414.


